Cardiac Dynamics
Description
- Modelling of atrial fibrillation. Computational analysis of the impact of genetic and clinical risk on molecular signaling and electrophysiological dysfunction in atrial fibrillation. Theoretical analysis of Ca homeostasis and Ca waves in cardiomyocytes. Methods: Computational models of cardiac function at the subcellular and tissue levels, developed and validated with experimental data from human atrial myocytes. Analysis of experimental images from confocal microscopy (also: I. CELL BIOPHYS and V. PATT REC IN BIOMED IM)
We are interested in understanding the transition to different types of arrhythmias. For that, we have developed models at the organ, tissue, cellular and sub cellular scales, focusing in electrical wave propagation, its coupling to contraction and the dynamics of intracellular calcium. This requieres the use of different numerical and analytical techniques, to analyse the resulting ordinary, parcial or stochastic equations.
Among the topics we are interested in are:
Cardiac alternans
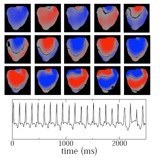
Contact: Blas Echebarria, Enric Alvarez
Modelling diffuse fibrosis
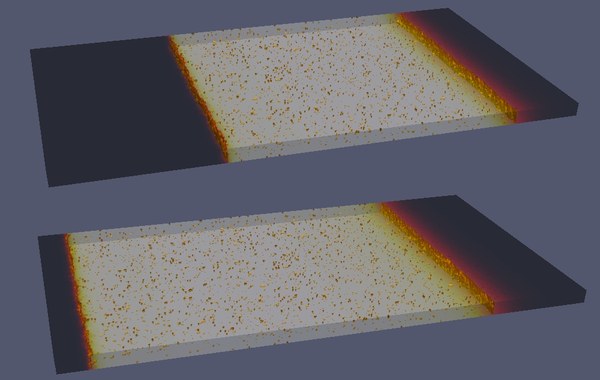
Contact: Sergio Alonso
Action potential wave instabilities

The propagation of the action potential through the tissue of the heart follows the cable equation, which can be interpreted as a complex reaction-diffusion system. While wave propagation is related to the usual electrical wave controlling the heart contraction, different types of more complex structures obtained from wave instabilities are related with cardiac re-entries, typically associated with cardiac arrhythmias. For the correct interpretation of such instabilities, reduction to simple models where the analytical studies are plausible, provides a good approach. We study different types of instabilities, including wave alternans, spiral wave breakup, and sproing and negative filament tension instabilities of three-dimensional scroll waves.
Contact: Blas Echebarria, Sergio Alonso
Calcium dynamics in myocytes

Calcium is the cellular messenger responsible for the contraction of the heart. In cardiac myocytes at rest most of it is stored in the sarcoplasmic reticulum (SR). During depolarization, calcium in the SR is released, as the channels in the SR membrane, the ryanodine receptors (RyR), open in response to the influx of calcium coming from the extracellular medium, in a process known as calcium induced calcium release (CICR). This produces a large increase in the calcium concentration in the cytosol, that reaches again its resting value under the action of the Na-Ca and SERCA pumps. Problems in calcium dynamics handling can give rise to a large number of pro-arrhythmic behaviors, such as electromechanical alternans, early and afterdepolarizations, etc. We are currently developing atrial and ventricular cell models to study the mechanisms responsible for these rhythms.
Contact: Blas Echebarria, Enric Alvarez, Inma R. Cantalapiedra
Excitation-contraction coupling
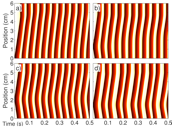
The increase of calcium in the cytosol during an action potential drives the contraction of the cardiac cell. When the concentration at the cytosol arrives at the μM level, the cross bridge cycle starts through the coupling of calcium to the protein complex troponim C (TnC), finally resulting in the generation of force by the muscle fiber. The coupling is not one-directional, though. At the cell membrane there are ion channels activated by stretch, such that deformation modifies the electrical properties of tissue. This provides a bidirectional coupling between wave propagation and tissue contraction that can have an important effect on the dynamics of action potential propagation, resulting, for instance, on the appearance of self-oscillatory regions of tissue, a modification of the dynamics of alternans or the appearance of different dynamical instabilities, such a spiral break-up.
Share: